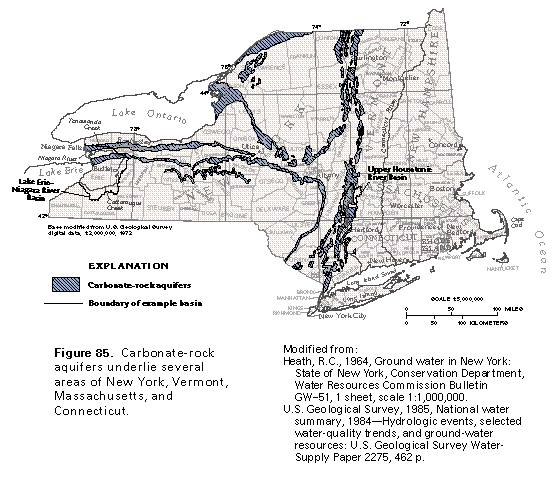
Carbonate Rock Aquifers originate as sedimentary deposits in marine environments. Compaction, cementation, and dolomitization processes (substitution of Mg for Ca) might act on the deposits as they lithify and greatly change their porosity and permeability. However, the principal post-depositional change in carbonate rocks is the dissolution of part of the rock by circulating, slightly acidic ground water. Solution openings in carbonate rocks range from small tubes and widened joints to caverns that may be tens of meters wide and hundreds to thousands of meters in length. Where they are saturated, carbonate rocks with well-connected networks of solution openings yield large amounts of water to wells that penetrate the openings, although the undissolved rock between the large openings may be almost impermeable. These areas are called karst.
There are two principal types of carbonate aquifers, therefore, fractured carbonate aquifers and and karstic carbonate aquifers.
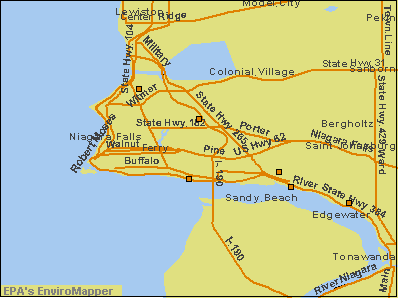
Carbonate rock is often fractured along bedding planes. These bedding planes are cross-cut by occasional sub-vertical joints, so that when they are saturated, the fractures form an interconnected network that store and transmit water. The bedrock aquifers of the Lake Erie-Niagara River Basin are an excellent local example of these types of aquifers.
Bedrock in the Lake Erie-Niagara River Basin consists chiefly of stratified limestone, dolomite, and shale of marine origin. These beds were formed when a large area west of the Appalachians was an inland sea. The surface increases in altitude to the south, in contrast to the dip of the rocks which is about 30-40 feet per mile. Thus, rocks that form the bedrock surface in parallel, east-northeast-trending belts are successively younger toward the south.
The bedrock units in the northern part of Lake Erie-Niagara River Basin have been divided into three aquifers (see below). The aquifers are not separated by confining units, but can be distinguished by their contrasting water-yielding characteristics. Except where they form the bedrock surface and are mostly covered by glacial deposits, the three aquifers are overlain and underlain by thick sequences of shale that form effective confining units. Conductivity of the bedrock units are controlled by the extent to which they have been fractured and dissolved by the action of water. The occurrence of gypsum beds can greatly increase conductivity.
The Lockport aquifer is of particular interest because it underlies the Niagara Falls area, which is one of the most heavily polluted urban areas in the United States. Six superfund sites lie directly over the Lockport.
Water is transmitted in the Lockport primarily in narrow zones associated with bedding fractures (joints), which in turn are often associated with lithologic contacts. The sparseness of these conductive zones makes monitoring of contamination problematic. Dense non-aqueous phase liquids (DNAPL) released to the formation tend to take circuitous routes downward through the fractures, making it even more difficult to find the non-dissolved phase of these contaminants.
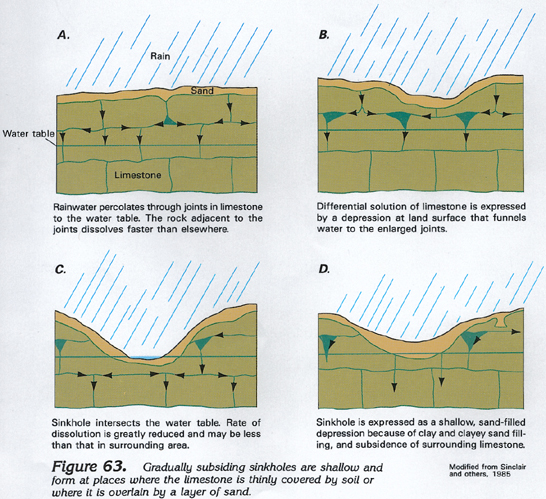
The most important current and future environmental issue with respect to karst is the sensitivity of karst aquifers to
groundwater contamination. The effect of man on karst is most
severe in cases where polluted surface waters enter karst aquifers. This problem is
universal among all karst regions in the United States that underlie populated areas.
Karstic aquifers can not filter contaminated groundwater sufficiently to render it
potable at a discharge sites. Water travels rapidly through solutional conduits because
recharge points are directly connected to discharge points. Not only does the "garbage
in, garbage out" principal apply to karstic groundwater, but contaminants move rapidly
and with relatively little dilution in this type of terrane.
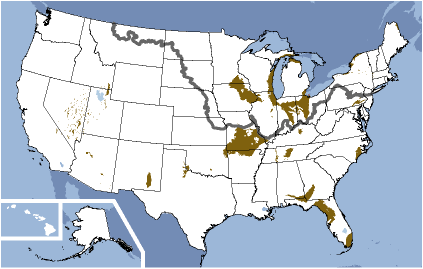
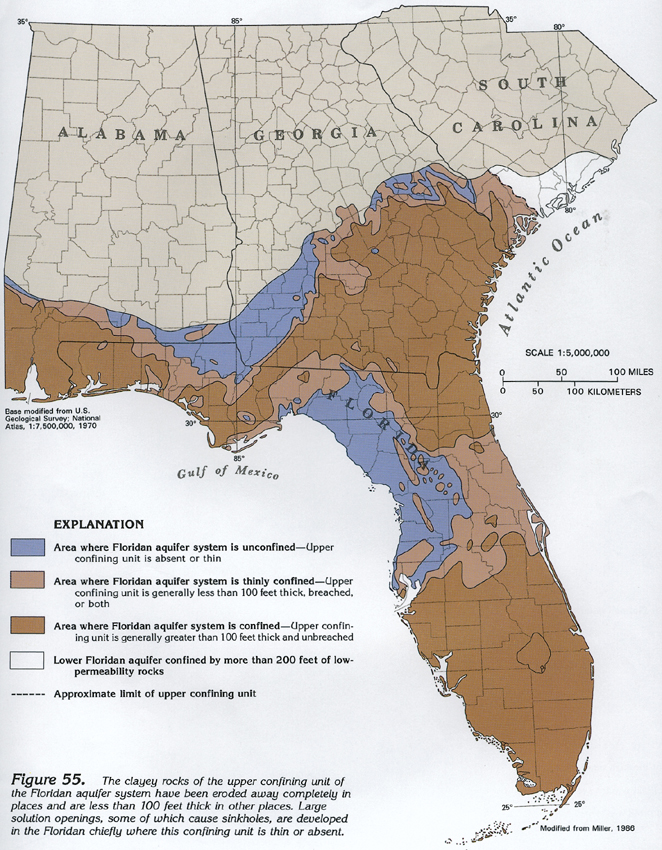
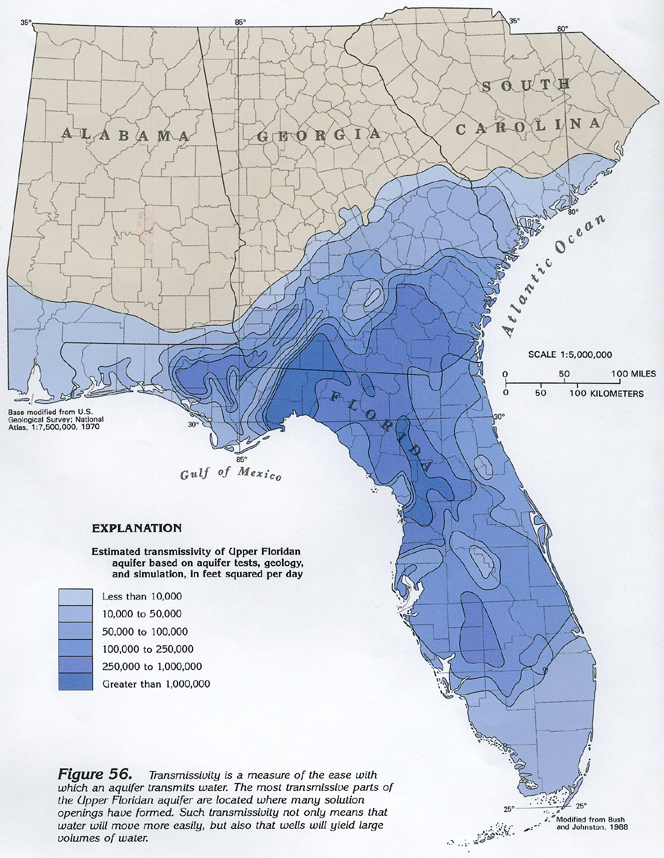
The Floridan Aquifer system is highly impacted by Karst Terrane and heavily utilized. In 1950, withdrawals of freshwater from the Floridan for all purposes totaled about 630 million gallons per day, by 1980, nearly five times this volume, or about 3 billion gallons per day, was being pumped. Over many regions, there is little or no confining material so that the carbonate rock is exposed to the surface, and therefore subject to dissolution. The map below shows that areas where the aquifer is unconfined correlates to areas of extremely high conductivity due to dissolution.
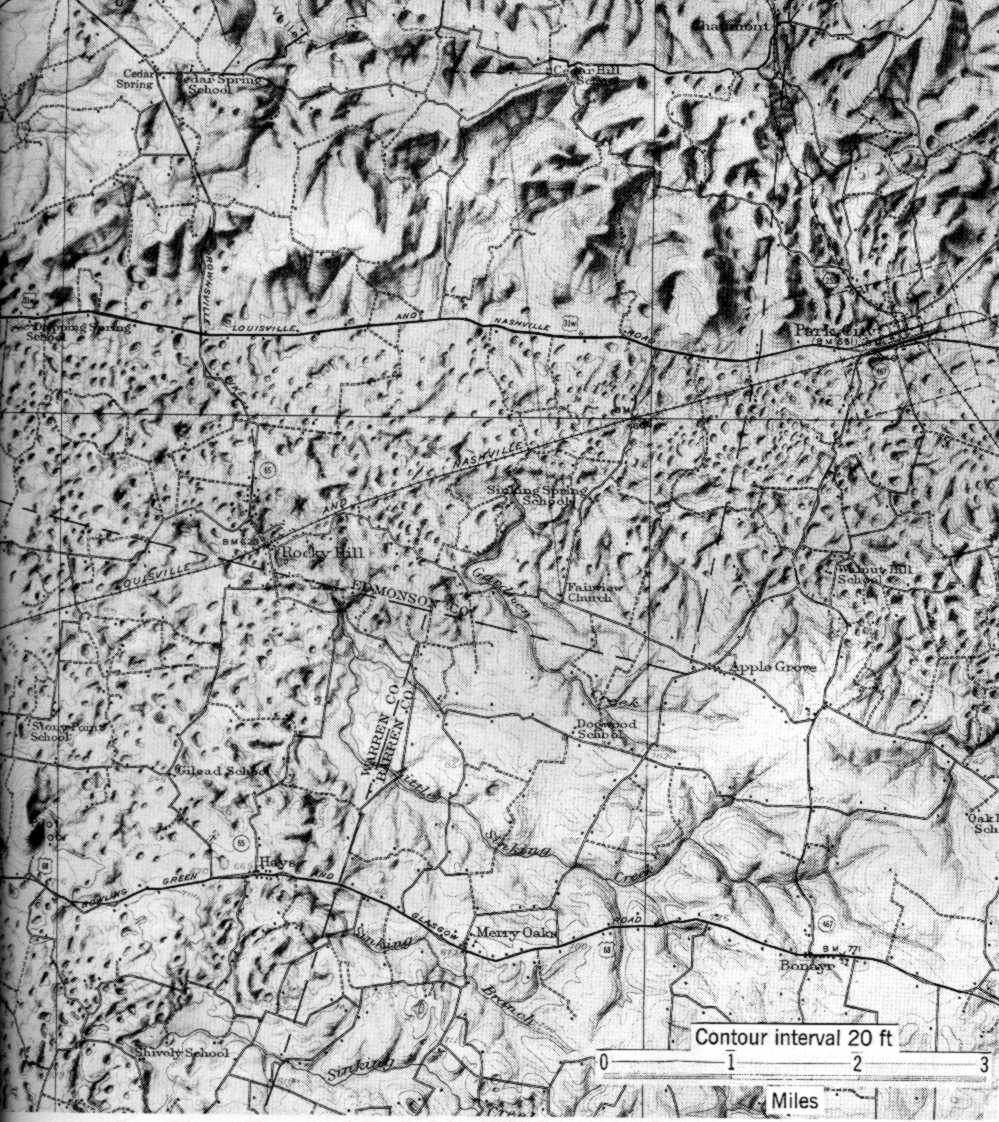
Karst terrane is often identifiable from a landscape marked with sinkholes. Below is a topographic map of karst features near Mammoth Cave, Kentucky. You may be able to get an air photo of the same region here.